Abstract
The aim of this study was to evaluate the effects of leukocyte- and platelet-rich fibrin (L-PRF) on the pain and soft tissue healing after tooth extractions. Twenty-six patients (9 males and 17 females) were treated with multiple extractions (2 to 8), with a total of 108 extractions. This was an exploratory single blinded randomized clinical trial with a split-mouth design. The pain after the surgery was assessed in each patient by the VAS scale (1 to 10) at intervals of 24-48-72-96 hours. The soft tissue healing was clinically evaluated at 3, 7, 14, and 21 days after surgery by the same examiner surgeon, using the modified Healing Index (4 to 12). The mean value of postextraction pain was 3.2 ± 0.3 in the experimental sides and 4.1 ± 0.1 in the control sides. After 7 days from the extractions, the values of modified Healing Index in the experimental and control groups were, respectively, 4.8 ± 0.6 and 5.1 ± 0.9. The use of L-PRF in postextraction sockets filling can be proposed as a useful procedure in order to manage the postoperative pain and to promote the soft tissue healing process, reducing the early adverse effects of the inflammation.
1. Introduction
Many studies revealed that platelet concentrates for surgical use can be used as efficient adjuvants for tissue repair [1–5]. The growth factors (particularly platelet-derived growth factors (PDGF), transforming growth factors (TGF-β), and vascular endothelial growth factors (VEGF)) and the other molecules (fibrinogen, fibronectin, and vitronectin) contained in platelets (α-granules) give to these products the ability to modulate many phases of the healing process like the hemostasis and the neoangiogenesis [6]. The clinical results of these products are interesting but remain quite mixed and controversial in the literature, depending on the kind of preparation [7–10]. Platelet concentrates are classified into 4 main families depending on their leukocyte and fibrin content: pure platelet-rich plasma (P-PRP), leukocyte- and platelet-rich plasma (L-PRP), pure platelet-rich fibrin (P-PRF), and leukocyte- and platelet-rich fibrin (L-PRF) [11]. Each family of products has different aspect, biological content, and potential application [12].
The PRPs were already tested in many oral surgery applications, with mixed results depending on the kind of preparations [13–16]. Numerous protocols have attempted to optimize the preparation of the autologous factors, using various performances standards and centrifugation parameters [17, 18]. Several authors demonstrated the effectiveness of some PRP types during tooth extractions to stimulate soft tissue healing and wound control [19, 20] and in prevention of postoperative bleeding in anticoagulated patients undergoing oral surgery. However, these PRP techniques remain quite complex and expensive on a daily use basis, and their use may not be justified for daily oral surgery applications [13, 14].
On the other hand, L-PRF represents a more recent generation of platelet concentrates. The development of L-PRF is very significant in oral and maxillofacial surgery, with many validated applications in periodontal surgery [13, 21] and implant dentistry [14–22]. L-PRF is easy and inexpensive to prepare for frequent use in private practice, and it exists in the form of L-PRF clots or membranes (after compression). The membrane releases a significant quantity of autologous growth factors (particularly PDGF-AB, TGFβ, and VEGF) [23], cytokines, and healing proteins (fibronectin, etc.) during more than 7 days in vitro [24], while other platelet gels dissolve in vitro in 3 days [12]. In another study, when compared with a procedure for platelet-rich plasma (PRP), L-PRF released more than 15-fold VEGF and more than 2-fold TGFβ1 [25]. According to the literature, L-PRF was a useful tool in postextraction hemostasis control [26] and in prevention of hemorrhagic complications in cardiopathic patients [27].
The aim of this study is to evaluate the effectiveness of L-PRF to improve the soft tissues healing and to reduce pain after tooth extractions.
2. Materials and Methods
2.1. Study Population
From January 2012 to July 2013 at the Unit of Oral Surgery and Implantology of the University of Naples “Federico II,” 26 healthy patients were selected, including 9 males and 17 females with a mean age of 53 ± 4 years. The selected patients were nonsmokers or light smokers (<5/day); they did not have systemic diseases that could interfere with the healing process (such as diabetes, liver disease, heart disease, or immune-disorders) or diseases of the oral mucosa. The study was designed as a prospective split-mouth trial on patients who needed bilateral paired dental extractions; on the side chosen to be the study side, the sockets were filled with L-PRF, whereas on the other side (control), they were allowed to undergo natural healing. Test and control sites were chosen with coin toss randomization. This cross-sectional split-mouth research study was conducted in accordance with the requirements of Helsinki Declaration of 1975 as revised in 2008. Patients were verbally informed about the sample to be taken and gave their written consent. The patients who did not sign the agreement and also the patients with poor oral hygiene, patients with local infections of the soft tissues, patients undergoing bisphosphonates therapy, patients irradiated to the jaws in the past, and patients with psychiatric illness or pregnant patients were excluded from the study.
2.2. Surgical Procedure
An alveolar nerve block infiltration was administrated with local or regional anesthesia, depending on the dental arch, using 2% mepivacaine. Mepivacaine does not contain epinephrine, so it was used to prevent restriction of the blood supply. To prevent interference with the healing process, no intraligamentous or intrapapillary infiltration was made. The teeth were extracted in a nontraumatic manner without elevation of full-thickness flaps and preserving the buccal and lingual walls of the alveolar sockets in order to minimize the possible trauma and to give adequate support to the L-PRF filling (Figures (Figures11 and and2).2). All extraction sites were simple with alveolar walls preserved. All alveolar sockets were sutured with a 3/0 Vicryl (Ethicon/Johnson & Johnson, Somerville, NJ, USA). Each patient also served as the control (split-mouth design): a socket was treated with L-PRF application (study socket) whereas the other (control socket) had to undergo natural healing by clot formation without socket filling (Figure 3). The number of paired extractions per patient ranged from 2 to 4 for a total of 108 extractions. Indication for tooth extraction included root or crow fractures, residual roots, no restorable caries, periapical granuloma, and orthodontic reasons. Patients showing anatomic and pathologic conditions not comparable between the study and control sites were excluded as study subject. Antibiotic prophylaxis was undertaken (amoxicillin 875 mg and clavulanic acid 125 mg) starting 2 days before surgery up to 3 days after it.
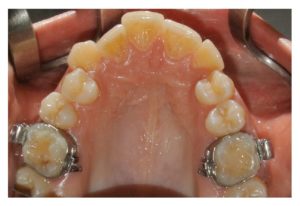
Figure 1
Presurgery clinical occlusal view. For orthodontic motive, this patient needed the bilateral extraction of the upper first premolars.
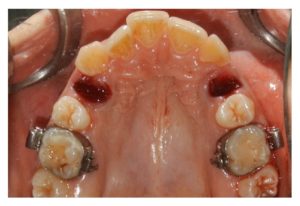
Figure 2
The postextraction sockets.
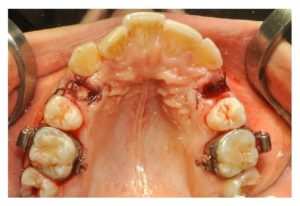
Figure 3
The upper right socket (study site) was filled with L-PRF, while the upper left socket (control site) had to follow natural healing. Both sites were sutured.
2.3. L-PRF Preparation Protocol
The L-PRF was prepared through a single centrifugation of blood according to the protocol of Dohan Ehrenfest et al. (now marketed as Intra-Spin L-PRF kit, Intra-Lock, Boca-Raton, FL, USA) for a period of 12 minutes at 2700 rpm. Blood was taken in 9 mL tubes, 30 minutes before the surgery, immediately centrifuged, and used for the filing of the experimental sites. The total amount of blood collected (from 18 mL to 54 mL) was related to the number of tooth extractions, in order to obtain the complete filling of the sockets with the L-PRF. After centrifugation, each L-PRF clot was separated from the portion of red blood cells (red thrombus), obtaining a fibrin clot with a red small portion in order to include the “buffy” coat richer in large leucocytes [24]. The L-PRF clot was condensed and modeled on a sterile surgical plate before the application in the sockets [24].
L-PRF was used within 60 minutes after the preparation. It was accurately positioned in the extraction sites and stabilized with a resorbable suture. In the control sites, the same suture was used (Figure 3). All patients were advised to follow soft and liquid diet, avoiding hot food in the following hours. In all cases, the sutures were removed after one week. Table 1 reports the number and the type of paired extractions per patient.
Table 1
| Number of patients | Number of paired extractions per patient | Tooth extracted |
|---|---|---|
| 10 | 1 | Premolar |
| 7 | 2 | Canine/premolar/molar |
| 6 | 3 | Canine/premolar/molar |
| 3 | 4 | Canine/premolar/molar |
2.4. Study Variables and Statistical Methods
The predictor variable was the treatment group status: L-PRF versus control socket. The outcome variables of interest were as follows: pain, postsurgical complications to soft and hard tissues, and the Healing Index modified.
A 10-point visual analog scale (VAS) with a score of 0 that equals “no pain” and a score of 10 that equals “very severe pain” was used by the same patient to assess the postoperative pain at 24, 48, and 72 hours. Between groups comparisons for VAS outcomes were carried out by means of univariate analysis of variance, considering the group (i.e., PRF versus CTR) and the recording time point as factors and VAS score as dependent variable.
The quality of the socket soft tissue healing was clinically evaluated at 3, 7, 14, and 21 days after surgery by an examiner surgeon, using the Healing Index modified [28] which involved 3 scoring levels for each of the four parameters considered: bleeding, suppuration, tissue color, and consistency of the healing tissue. The scoring scale ranged from 4, corresponding to excellent healing, to 12, indicating severely impaired healing. The Wilcoxon signed-rank test for comparison of 2 correlated samples matched pairs with the level of significance predetermined at 0.05 was used.
3. Results
All patients completed the study. No cases of bleeding, infection, alveolar osteitis, or other surgical complications were reported.
Regarding the postextraction pain, patients enrolled in the study reported a mean value of the study sites of 3.2 ± 0.3, which is lower (P < 0.0001) than the mean value of the control sites (4.5 ± 0.7), with a statistical difference average of 0.9 ± 0.3. The VAS score was nearly equal for the 2 sides after 4 days (decreasing to 0).
Results concerning the healing of the socket are reported in Table 2. Comparisons between values relative to the study and control sides showed better healing and faster socket closure for the side treated with L-PRF, with differences statistically significant at days 3 and 7 (Figures (Figures44 and and55).
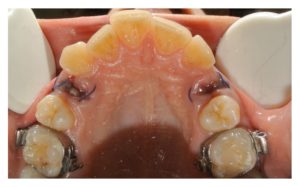
Figure 4
Clinical occlusal view 3 days after surgery. In the study site, the epithelialization process was more advanced than in the control site. In the study site, the inflammatory reaction was reduced.
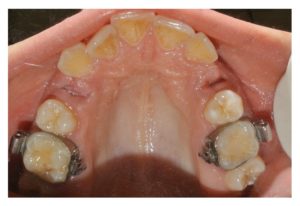
Figure 5
Clinical occlusal views 7 days after surgery. The sutures were removed. Both postextraction socket cavities presented a decreased volume and appeared epithelialized.
Table 2
| Healing Index | 3 days | 7 days | 14 days | 21 days |
|---|---|---|---|---|
| L-PRF | 4.8 ± 0.6 | 4.5 ± 0.5 | 4.2 ± 0.2 | 4.1 ± 0.1 |
| CTRL | 5.1 ± 0.9 | 4.9 ± 0.3 | 4.3 ± 0.3 | 4.2 ± 0.2 |
| P | 0.197 | 0.05 | 0.01 | 0.0002 |
L-PRF = study site; CTRL = control site.
4. Discussion
This study was designed to test the efficacy of L-PRF in fostering socket healing after tooth extractions. The 26 split-mouth case control extractions that constituted our study were statistically enough to prove the ability of L-PRF to improve the early healing phases (hemostasis and epithelial closure), reducing the inflammatory process and the risk of infection. The reported results of the experimental sites showed, in the first 7 days after the tooth extractions, a fast evolution of the healing and a positive effect on pain. After a week, minor differences between the two groups are reported (Figures (Figures66 and and7).7). These effects could be related to the biochemical and structural features of the L-PRF [29], which collects a large quantity of leukocytes (about 60% of the initial blood harvest) and platelets embedded in a fibrin matrix [30, 31]. The fibrin architecture of L-PRF, constituted by connected trimolecular junctions (or equatorial), due to a slow polymerization of the platelet concentrate and due to the absence of heterologous thrombin, induces a flexible fibrin network, able to promote the gradual release of growth factors and leukocytes migration. The fibrin membrane promotes the mechanical protection of the surgical site and, biologically, it interacts with the physiological mechanisms of healing favoring the angiogenesis [13, 14]. The fibrin induces the expression of αv-β3 integrin by endothelial cells, allowing the links with structural proteins, such as fibronectin and vitronectin, supporting the process of formation of capillaries [12]. In relation to the previous properties, the fibrin also allows the association of some growth factors, such as FGFb (fibroblast growth factor basic) and PDGF (platelet-derived growth factor) involved in the angiogenic process and useful as chemotactic factors, favoring diapedesis of white blood cells [12]. The immunological properties of the L-PRF, resulting from its content in leukocytes, could be useful to prevent the surgical site infections, such as postextraction alveolitis, with a consequent reduction of the inflammation symptoms. The presence of leukocytes is a very important parameter to stimulate healing and wound control [32].
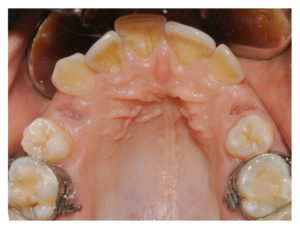
Figure 6
Clinical follow-up at 14 days after surgery. Both postextraction sockets were completely closed with the soft tissues.
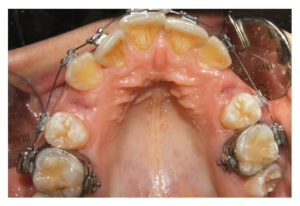
Figure 7
At 21 days after surgery, both postextraction healing sites were completely closed with the soft tissues.
The main limitation of this exploratory study was that the extraction sites were voluntarily very simple, with all alveolar walls preserved. It allowed standardizing the study easily to reach a very clean result, but it does not reflect the real strength and advantages of L-PRF. This material is particularly useful and efficient in complex situations, when some walls are destroyed and the bone regeneration is difficult, but an accurate split-mouth study with this kind of cases is virtually impossible to standardize. It is however the needed next step of evaluation and validation of the use of L-PRF during tooth extractions.
5. Conclusion
Even if the selected samples are limited, the reported results suggested that the use of L-PRF in postextraction sockets filling is an efficient and useful procedure in order to manage the postoperative pain and to enhance the alveolar soft tissue healing process, especially in the first days after the extractions, reducing the early adverse effects of the inflammation. This study represents a preliminary clinical trial, which could be used as baseline for further histologic studies.
Conflict of Interests
The authors have no conflict of interests to declare regarding the devices used for this study. The current research was not influenced by any secondary interests, such as financial gain.
References
1. Bielecki T., Dohan Ehrenfest D. M. Leukocyte- and platelet-rich Plasma (L-PRP)/fibrin (L-PRF) in medicine—past, present, future. Current Pharmaceutical Biotechnology. 2012;13(7):1–2. doi: 10.2174/138920112800624274. [PubMed] [Cross Ref]
2. Gruber R., Varga F., Fisher M. B., Watzek G. Platelet stimulate proliferation of bone cells:involvement of platelet-derived growth factor, microparticles and membranes. Clinical Oral Implants Research. 2002;13:529–535. [PubMed]
3. Carlson N. E., Roach J. R. B. Platelet-rich plasma: clinical application in dentistry. The Journal of the American Dental Association. 2002;133:1383–1386. [PubMed]
4. Sanchez A. R., Sheridan P. J., Kupp L. I. Is platelet-rich plasma the perfect enhancement factor? A current review. The International Journal of Oral & Maxillofacial Implants. 2003;18:93–103. [PubMed]
5. Anitua E., Andia I., Ardanza B., et al. Autologous platelets as a sourge of proteins for healing and tissue regeneration. Thrombosis and Haemostasis. 2004;91:4–15. [PubMed]
6. Marx R. E., Carlson E. R., Eichstaedt R. M., Schimmele S. R., Strauss J. E., Georgeff K. R. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 1998;85(6):638–646. doi: 10.1016/s1079-2104(98)90029-4. [PubMed] [Cross Ref]
7. Everts P. A. M., Hoogbergen M. M., Weber T. A., Devilee R. J. J., van Monftort G., de Hingh I. H. J. T. Is the use of autologous platelet-rich plasma gels in gynecologic, cardiac, and general, reconstructive surgery beneficial? Current Pharmaceutical Biotechnology. 2012;13(7):1163–1172. doi: 10.2174/138920112800624346. [PubMed] [Cross Ref]
8. Yuan T., Guo S.-C., Han P., Zhang C. Q., Zeng B. F. Applications of leukocyte- and platelet-rich plasma (L-PRP) in trauma surgery. Current Pharmaceutical Biotechnology. 2012;13(7):1173–1184. doi: 10.2174/138920112800624445. [PubMed] [Cross Ref]
9. del Fabbro M., Bortolin M., Tascheri S. Is autologous platelet concentrate beneficial for post-extraction socket healing? A systematic review. International Journal of Oral and Maxillofacial Surgery. 2011;40:891–900. [PubMed]
10. Esposito M., Grusovin M. G., Rees J., et al. Effectiveness of sinus lift procedures for dental implant rehabilitation: a Cochrane systematic review. European Journal of Oral Implantology. 2010;3:7–26. [PubMed]
11. Dohan Ehrenfest D. M., Sammartino G., Shibli J. A., Wang H. L., Zou D. R., Bernard J. P. Guidelines for the publication of articles related to platelet concentrates (Platelet-Rich Plasma—PRP, or Platelet-Rich Fibrin—PRF): the international classification of the POSEIDO. POSEIDO. 2013;1(1):17–27.
12. Dohan Ehrenfest D. M., Bielecki T., Jimbo R., et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasm (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF) Current Pharmaceutical Biotechnology. 2012;13(7):1145–1152. doi: 10.2174/138920112800624382. [PubMed] [Cross Ref]
13. del Corso M., Vervelle A., Simonpieri A., et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: periodontal and dentoalveolar surgery. Current Pharmaceutical Biotechnology. 2012;13(7):1207–1230. doi: 10.2174/138920112800624391. [PubMed] [Cross Ref]
14. Simonpieri A., del Corso M., Vervelle A., et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery. Part 2. Bone graft, implant and reconstructive surgery. Current Pharmaceutical Biotechnology. 2012;13(7):1231–1256. doi: 10.2174/138920112800624472. [PubMed] [Cross Ref]
15. Geurs N., Ntounis A., Vassilopoulos P., van der Velden U., Loos B. G., Reddy M. Using growth factors in human extraction sockets: a histologic and histomorphometric evaluation of short-term healing. The International Journal of Oral & Maxillofacial Implants. 2014;29(2):485–496. doi: 10.11607/jomi.3408. [PubMed] [Cross Ref]
16. Kaur P., Maria A. Efficacy of platelet rich plasma and hydroxyapatite crystals in bone regeneration after surgical removal of mandibular third molars. Journal of Maxillofacial and Oral Surgery. 2013;12(1):51–59. doi: 10.1007/s12663-012-0382-6. [PMC free article] [PubMed] [Cross Ref]
17. Weibrich G., Kleis W. K. G., Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. International Journal of Oral & Maxillofacial Implants. 2002;17(2):184–190. [PubMed]
18. Borzini P., Balbo V., Mazzucco L. Platelet concentrates for topical use: bedside device and blood transfusion technology. Quality and versatility. Current Pharmaceutical Biotechnology. 2012;13(7):1138–1144. doi: 10.2174/138920112800624454. [PubMed] [Cross Ref]
19. Rivera C., Monsalve F., Salas J., Morán A., Suazo I. Platelet-rich plasma, plasma rich in growth factors and simvastatin in the regeneration and repair of alveolar bone. Experimental and Therapeutic Medicine. 2013;6(6):1543–1549. doi: 10.3892/etm.2013.1327. [PMC free article] [PubMed] [Cross Ref]
20. Sammartino G., Tia M., Bucci T., Wang H. L. Prevention of mandibular third molar extraction-associated periodontal defects: a comparative study. Journal of Periodontology. 2009;80(3):389–396. [PubMed]
21. Chang Y. C., Zhao J. H. Effect of platelet-rich fibrin on human periodontal ligament fibroblasts and application forperiodontal infrabony defects. Australian Dental Journal. 2011;56:365–371. [PubMed]
22. Toeroek R., Dohan Ehrenfest D. M. The concept of Screw-Guided Bone Regeneration (S-GBR). Part 3: fast screw-guided bone regeneration (FS-GBR) in the severely resorbed preimplant posterior mandible using allograft and leukocyte- and platelet-rich fibrin (L-PRF): a 4-year follow-up. POSEIDO. 2013;1(2):93–100.
23. Zumstein M. A., Berger S., Schober M., et al. Leukocyte- and platelet-rich fibrin (L-PRF) for long-term delivery of growth factor in rotator cuff repair: review, preliminary results and future directions. Current Pharmaceutical Biotechnology. 2012;13(7):1196–1206. doi: 10.2174/138920112800624337. [PubMed] [Cross Ref]
24. Dohan Ehrenfest D. M., de Peppo G. M., Doglioli P., Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63–69. [PubMed]
25. Passaretti F., Tia M., D’esposito V., et al. Growth-promoting action and growth factor release by different platelet derivatives. Platelets. 2014;25(4):252–256. doi: 10.3109/09537104.2013.809060. [PubMed] [Cross Ref]
26. Dohan Ehrenfest D. M., Vazquez L. Pulling out, extraction or avulsion? Implant Dentistry. 2008;17(1):p. 4. doi: 10.1097/id.0b013e31816760b4. [PubMed] [Cross Ref]
27. Sammartino G., Dohan Ehrenfest D. M., Carile F., Tia M., Bucci P. Prevention of hemorrhagic complications after dental extractions into open heart surgery patients under anticoagulant therapy: the use of leukocyte- and platelet-rich fibrin. The Journal of Oral Implantology. 2011;37(6):681–690. doi: 10.1563/aaid-joi-d-11-00001. [PubMed] [Cross Ref]
28. Mozzati M., Gallesio G., di Romana S., Bergamasco L., Pol R. Efficacy of plasma-rich growth factor in the healing of postextraction sockets in patients affected by insulin-dependent diabetes mellitus. Journal of Oral and Maxillofacial Surgery. 2014;72(3):456–462. doi: 10.1016/j.joms.2013.10.010. [PubMed] [Cross Ref]
29. Dohan Ehrenfest D. M., del Corso M., Inchingolo F., Charrier J. B. Selecting a relevant in vitro cell model for testing and comparing the effects of a Choukroun’s platelet-rich fibrin (PRF) membrane and a platelet-rich plasma (PRP) gel: tricks and traps. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2010;110(4):409–413. doi: 10.1016/j.tripleo.2010.05.056. [PubMed] [Cross Ref]
30. Choukroun J., Diss A., Simonpieri A., et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2006;101:56–60. [PubMed]
31. Kang Y.-H., Jeon S. H., Park J.-Y. Platelet-rich fibrin is a bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Engineering Part A. 2011;17:349–359. [PubMed]
32. Bielecki T., Dohan Ehrenfest D. M., Everts P. A., Wiczkowski A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Current Pharmaceutical Biotechnology. 2012;13(7):1153–1162. doi: 10.2174/138920112800624373. [PubMed] [Cross Ref]
Articles from BioMed Research International are provided here courtesy of Hindawi Limited



